Overview
The main goals of our research efforts are to understand what causes epilepsy and to develop new treatments for this devastating disease. Below are a number of ongoing projects in the lab:
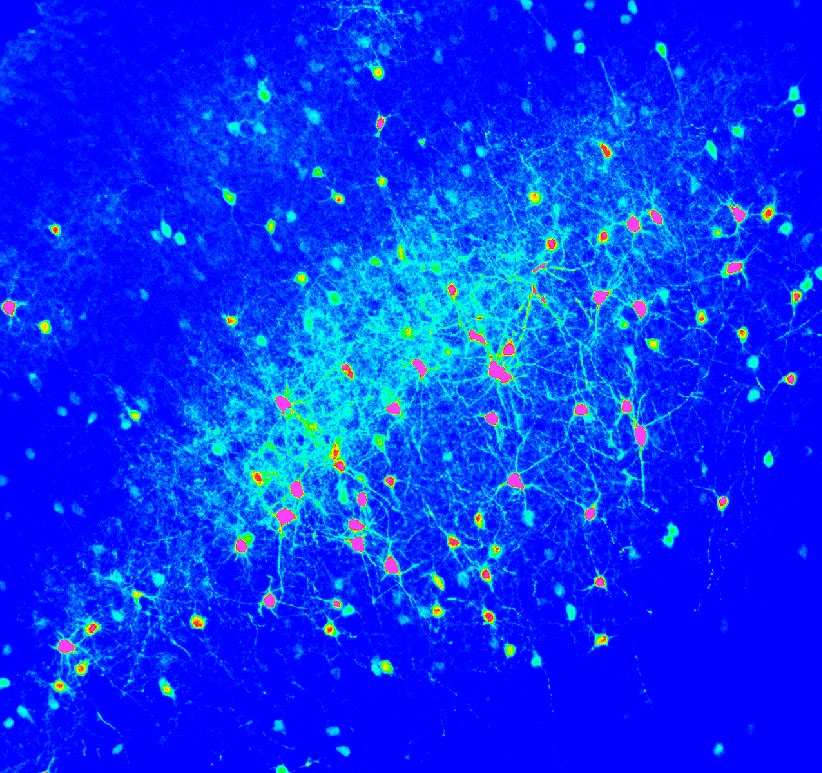
The Role of Glutamate in Brain DevelopmentThe control of neurotransmitter release is a highly regulated process, as is the fate of neurotransmitter once it is released from the presynaptic terminal. Each time NT is released a transient occurs consisting of a rapid rise in the extracellular concentration of neurotransmitter followed by a return to baseline concentration. We aim to understand how glutamate activity drives cortical network maturation and how astrocyte glutamate uptake provides spatio-temporal control of neurotransmitter transients. We suspect that disruption of glutamate signaling in the developing cortex can lead to pathological disease states such as epilepsy. Our previous work has shown that the maintenance of stereotyped glutamate transients ensures proper neuronal network function and if perturbed can lead to pathological, epileptic network activity. The specific questions we aim to answer are: 1) how does glutamate reuptake shape NT transients in the developing brain, 2) how does neonatal brain injury alter NT transients, and 3) can these changes contribute to the progression of diseases such as epilepsy.
|
Traumatic Brain Injury
|
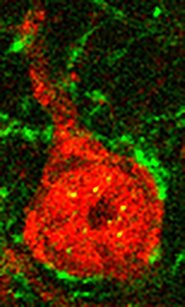
Traumatic brain injury (TBI) causes cognitive and motor dysfunction and greatly increases the risk of developing epilepsy. Recent increases in head trauma due to sports, military, and automobile related injuries have increased the need to better understand the cellular and molecular changes which occur following brain trauma and to develop novel treatment strategies. Following TBI, inhibitory GABAergic interneurons are lost. These cells are essential to controlling network function and their loss may be linked to epilepsy. We have begun experiments which will allow us to pinpoint regions of interneuron loss following brain injury using cutting edge imaging and electrophysiological technologies. Our studies will determine whether interneuron loss or dysfunction contribute to pathological brain activity.
|
Activity-dependent Regulation of Glutamate Uptake
|
We recently reported that neuronal activity alters the rate of astrocytic glutamate uptake with spatial and temporal specificity. This suggests that astrocytes may be able to control glutamate signaling in a new way, with synapse specificity. If correct, this would have significant implications on how we view neuron-astrocyte interactions. We have now begun to study the mechanisms behind this modulation. We suspect that rapid regional changes in astrocyte voltage drive this important, novel type of synaptic plasticity. We are developing novel imaging approaches to understand these changes and dive deeper into the ions and molecules at play.
|
Infantile Spasms
|
Infantile spasms (IS) constitute a catastrophic childhood epilepsy syndrome. The pathophysiologic changes that cause IS are poorly defined but their identification is critical to developing effective therapeutic interventions. Animal models of IS are required to accomplish this goal, but remain sparse. We have developed a new a mouse model with a conditional deletion of the adenomatous polyposis coli (APC) gene in excitatory neurons (APC cKO). APC cKO mice recapitulate the salient features of human IS including neonatal behavioral spasms, abnormal EEG activity, and adult seizures. APC is a negative regulator of Wnt signaling and its deletion leads to excessive levels of b-catenin which is known to have dual functions in the N-cadherin synaptic adhesion complex and the canonical Wnt signaling pathway. Deregulation of both networks in the developing brain leads to altered axon guidance cues, excessive branching, aberrant density and plasticity of excitatory synapses, and changes in circuit connectivity, all consistent with increased seizure susceptibility. This project is a joint venture between the Dulla Lab and the lab of Michele Jacob.
|